Classification Of Matter
A substance is a sample of matter whose physical and chemical properties are the same throughout the sample because the matter has a constant composition. It is common to see substances changing from one state of matter to another. To differentiate the states of matter at least at a particle level, we look at the behavior of the particles within the substance. When substances change state, it is because the spacing between the particles of the substances is changing due to a gain or loss of energy. For example, we all have probably observed that water can exist in three forms with different characteristic ways of behaving: the solid state (ice), liquid state (water), and gaseous state (water vapor and steam). Due to water's prevalence, we use it to exemplify and describe the three different states of matter. As ice is heated and the particles of matter that make up water gain energy, eventually the ice melts in to water that eventually boils and turns into steam. Classifying Matter
States of MatterSolids
LiquidsGases
States of MatterSolids
LiquidsGases
Before we examine the states of matter, we will consider some ways samples of matter have been classified by those who have studied how matter behaves.
Evidence suggests that substances are made up of smaller particles that are ordinarily moving around. Some of those particles of matter can be split into smaller units using fairly strong heat or electricity into smaller rather uniform bits of matter called atoms. Atoms are the building blocks of elements. Elements are all those substances that have not ever been decomposed or separated into any other substances through chemical reactions, by the application of heat, or by attempting to force an direct electric current through the sample. Atoms in turn have been found to be made up of yet smaller units of matter called electrons, protons, and neutrons.
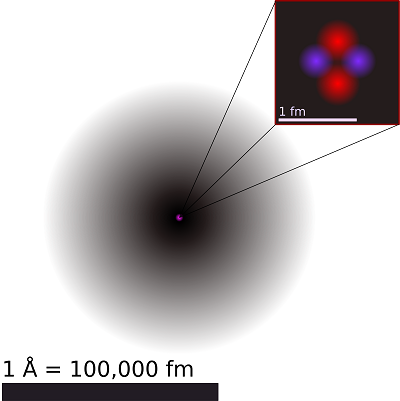
Figure 1: Breakdown of an atom. An illustration of the helium atom, depicting the nucleus (pink) and the electron cloud distribution (black). The nucleus (upper right) in helium-4 is in reality spherically symmetric and closely resembles the electron cloud, although for more complicated nuclei this is not always the case. The black bar is one angstrom (10−10 m or 100 pm). Image used with permissin from Wikipedia.
Elements can be arranged into what is called the periodic table of elements based on observed similarities in chemical and physical properties among the different elements. When atoms of two or more elements come together and bond, a compound is formed. The compound formed can later be broken down into the pure substances that originally reacted to form it.
Compounds such as water are composed of smaller units of bonded atoms called molecules. Molecules of a compound are composed of the same proportion of elements as the compound as a whole since they are the smallest units of that compound. For example, every portion of a sample of water is composed of water molecules. Each water molecule contains two hydrogen atoms and one oxygen atom, and so water as a whole has, in a combined state, twice as many hydrogen atoms as oxygen atoms..
Water can still consist of the same molecules, but its physical properties may change. For instance, water at a temperature below 0° Celsius (32° Fahrenheit) is ice, whereas water above the temperature of 100° C (212° F) is a gas, water vapor. When matter changes from one state to another, temperature and pressure may be involved in the process and the density and other physical properties change. The temperature and pressure exerted on a sample of matter determines the resulting form of that the matter takes, whether solid, liquid, or gas.
Since the properties of compounds and elements are uniform, they are classified as substances. When two or more substances are mixed together, the result is called a mixture. Mixtures can be classified into two main categories: homogeneous and heterogeneous. A homogeneous mixture is one in which the composition of its constituents are uniformly mixed throughout. A homogeneous mixture in which on substance, the solute, dissolves completely in another substance, the solvent, may also be called a solution. Usually the solvent is a liquid, however the solute can be either a liquid, solid, or a gas. In a homogeneous solution, the particles of solute are spread evenly among the solvent particles and the extremely small particles of solute cannot be separated from the solvent by filtration through filter paper because the spaces between paper fibers are much greater than the size of the solute and solvent particles. Other examples of homogeneous mixtures include sugar water, which is the mixture of sucrose and water, and gasoline, which is a mixture of dozens of compounds.
Example 1: Homogeneous Mixture
Filtered seawater is solution of the compounds of water, salt (sodium chloride), and other compounds.
Everything that is familiar to us in our daily lives - from the land we walk on, to the water we drink and the air we breathe - is based upon the states of matter called gases, liquids, and solids.
When the temperature of a liquid is lowered to the freezing point of the substance (for water the freezing point is 0oC), the movement of the particles slows with the spacing between the particles changing until the attractions between the particles lock the particles into a solid form. At the freezing point, the particles are closely packed together and tend to block the motions of each other. The attractions between the particles hold the particles tightly together so that the entire ensemble of particles takes on a fixed shape. The volume of the solid is constant and the shape of a solid is constant unless deformed by a sufficiently strong external force. (Solids are thus unlike liquids whose particles are slightly less attracted to one another because the particles of a liquid are a bit further apart than those in the corresponding solid form of the same substance.) In a solid the particles remain in a relatively fixed positions but continue to vibrate. The vibrating particles in a solid do not completely stop moving and can slowly move into any voids that exist within the solid.
When the temperature of a sample increases above the melting point of a solid, that sample can be found in the liquid state of matter. The particles in the liquid state are much closer together than those in the gaseous state, and still have a quite an attraction for each other as is apparent when droplets of liquid form. In this state, the weak attractive forces within the liquid are unable to hold the particles into a mass with a definite shape. Thus a liquid's shape takes on the shape of any particular container that holds it. A liquid has a definite volume but not a definite shape. Compared to to the gaseous state there is less freedom of particle movement in the liquid state since the moving particles frequently are colliding with one another, and slip and slide over one another as a result of the attractive forces that still exist between the particles, and hold the particles of the liquid loosely together. At a given temperature the volume of the liquid is constant and its volume typically only varies slightly with changes in temperature.
In the gas phase, matter does not have a fixed volume or shape. This occurs because the molecules are widely separated with the spaces between the particles typically around ten times further apart in all three spatial directions, making the gas around 1000 times less dense than the corresponding liquid phase at the same temperature. (A phase is a uniform portion of mater.) As the temperature of a gas is increased, the particles to separate further from each other and move at faster speeds. The particles in a gas move in a rather random and independent fashion, bouncing off each other and the walls of the container. Being so far apart from one another, the particles of a real gas only weakly attract each other such that the gas has no ability to have a shape of its own. The extremely weak forces acting between the particles in a gas and the greater amount of space for the particles to move in results in almost independent motion of the moving, colliding particles. The particles freely range within any container in which they are put, filling its entire volume with the net result that the sides of the container determine the shape and volume of gas. If the container has an opening, the particles heading in the direction of the opening will escape with the result that the gas as a whole slowly flows out of the container.
Pure Substances
Most forms of matter that we encounter—for example, the air we breathe (a gas), gasoline for cars (a liquid), and the sidewalk on which we walk (a solid)—are not chemically pure. We can, however, resolve, or separate, these kinds of matter into different pure substances. A pure substance (usually referred to simply as a substance) is matter that has distinct properties and a composition that doesn't vary from sample to sample. Water and ordinary table salt (sodium chloride), the primary components of seawater, are examples of pure substances.
All substances are either elements or compounds. Elements cannot be decomposed into simpler substances. On the molecular level, each element is composed of only one kind of atom [Figure 1.5 (a and b)]. Compounds are substances composed of two or more elements, so they contain two or more kinds of atoms [Figure 1.5(c)]. Water, for example, is a compound composed of two elements, hydrogen and oxygen. Figure 1.5(d) shows a mixture of substances. Mixtures are combinations of two or more substances in which each substance retains its own chemical identity.
Figure 1.5 Each element contains a unique kind of atom. Elements might consist of individual atoms, as in (a), or molecules, as in (b). Compounds contain two or more different atoms chemically joined together, as in (c). A mixture contains the individual units of its components, shown in (d) as both atoms and molecules.
Elements
At the present time 114 elements are known. These elements vary widely in their abundance, as shown in Figure 1.6. For example, only five elements account for over 90% of the Earth's crust: oxygen, silicon, aluminum, iron, and calcium. In contrast, just three elements (oxygen, carbon, and hydrogen) account for over 90% of the mass of the human body.
Compounds
Most elements can interact with other elements to form compounds. Hydrogen gas, for example, burns in oxygen gas to form water. Conversely, water can be decomposed into its component elements by passing an electrical current through it, as shown in Figure 1.7. Pure water, regardless of its source, consists of 11% hydrogen and 89% oxygen by mass. This macroscopic composition corresponds to the molecular composition, which consists of two hydrogen atoms combined with one oxygen atom.
Mixtures
Most of the matter we encounter consists of mixtures of different substances. Each substance in a mixture retains its own chemical identity and hence its own properties. Whereas pure substances have fixed compositions, the compositions of mixtures can vary. A cup of sweetened coffee, for example, can contain either a little sugar or a lot. The substances making up a mixture (such as sugar and water) are called components of the mixture.
Some mixtures, such as sand, rocks, and wood, do not have the same composition, properties, and appearance throughout the mixture. Such mixtures are heterogeneous . Mixtures that are uniform throughout are homogeneous. Air is a homogeneous mixture of the gaseous substances nitrogen, oxygen, and smaller amounts of other substances. The nitrogen in air has all the properties that pure nitrogen does because both the pure substance and the mixture contain the same nitrogen molecules. Salt, sugar, and many other substances dissolve in water to form homogeneous mixtures . Homogeneous mixtures are also called solutions.
are elements and subtance same?
BalasHapusNo, elements and subtance are different.
HapusSubstance is a material form that has a constant chemical composition and characteristic properties.
Elements are chemicals that are composed of certain types of atoms and therefore can not be broken down or changed by chemical reactions to different elements.
Explain difference of liquid,gas and solid!
BalasHapusThanks for your question.
HapusThe different of liquid, gas and solid:
Liquid has a certain volume, but does not have a fixed shape, depending on the media used, the distance between the particles of liquid is more tenuous. Liquid particles can move freely but are limited
Gas does not have a certain volume and shape. The distance between the particles of gas is very tenuous. The gas particles can move very freely.
Solid have a certain shape and volume. The distance between solid particles is very tight. The solid particles can not move
Why every elements have different atom number?
BalasHapusBecause atoms have different numbers of protons and electrons
HapusCan you explain the elemental difference with the diatomic element?
BalasHapusDiatomics is a molecule consisting of only two atoms. Both atoms can be the same or different elements. The prefix in diatomic word comes from the Greek meaning two. The elements found in the form of a diatomic molecule include hydrogen (H2), nitrogen (N2), oxygen (O2), and halogen: fluorine (F2), chlorine (Cl2), bromine (Br2), iodine (I2), and astatin (At2).
HapusThe element is an inseparable single substance to be simpler, simpler, ordinary chemical reactions. Example: Barium (Ba), Fluorine (F).
Please give me the examples for pure substances?
BalasHapus@hudiaumamifaisal
Water and ordinary table salt (sodium chloride), the primary components of seawater, are examples of pure substances.
HapusHere why Each element contains a unique atomic type?
BalasHapusBecause each element consists of two or more different atoms
HapusPlease explain about the states of matter
BalasHapusSubstances are examples of materials whose physical and chemical properties are the same throughout the sample because the material has a constant composition. Give an example that has a constant composition
BalasHapusIn chemistry, chemistry is a form of matter that has a constant chemical composition and characteristic properties. Some examples are diamond (carbon), gold, table salt (sodium chloride), and refined sugar (sucrose).
HapusWhether impure substance can be pure? If you can give an example?
BalasHapusThe substances making up a mixture (such as sugar and water) are called components of the mixture. Give more example in chemical?
BalasHapus